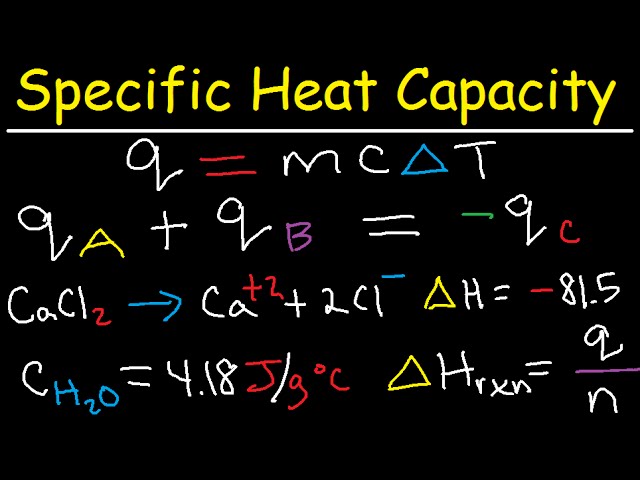
What is Specific Heat Capacity? | How to Find Specific Heat Capacity - Video & Lesson Transcript | Study.com
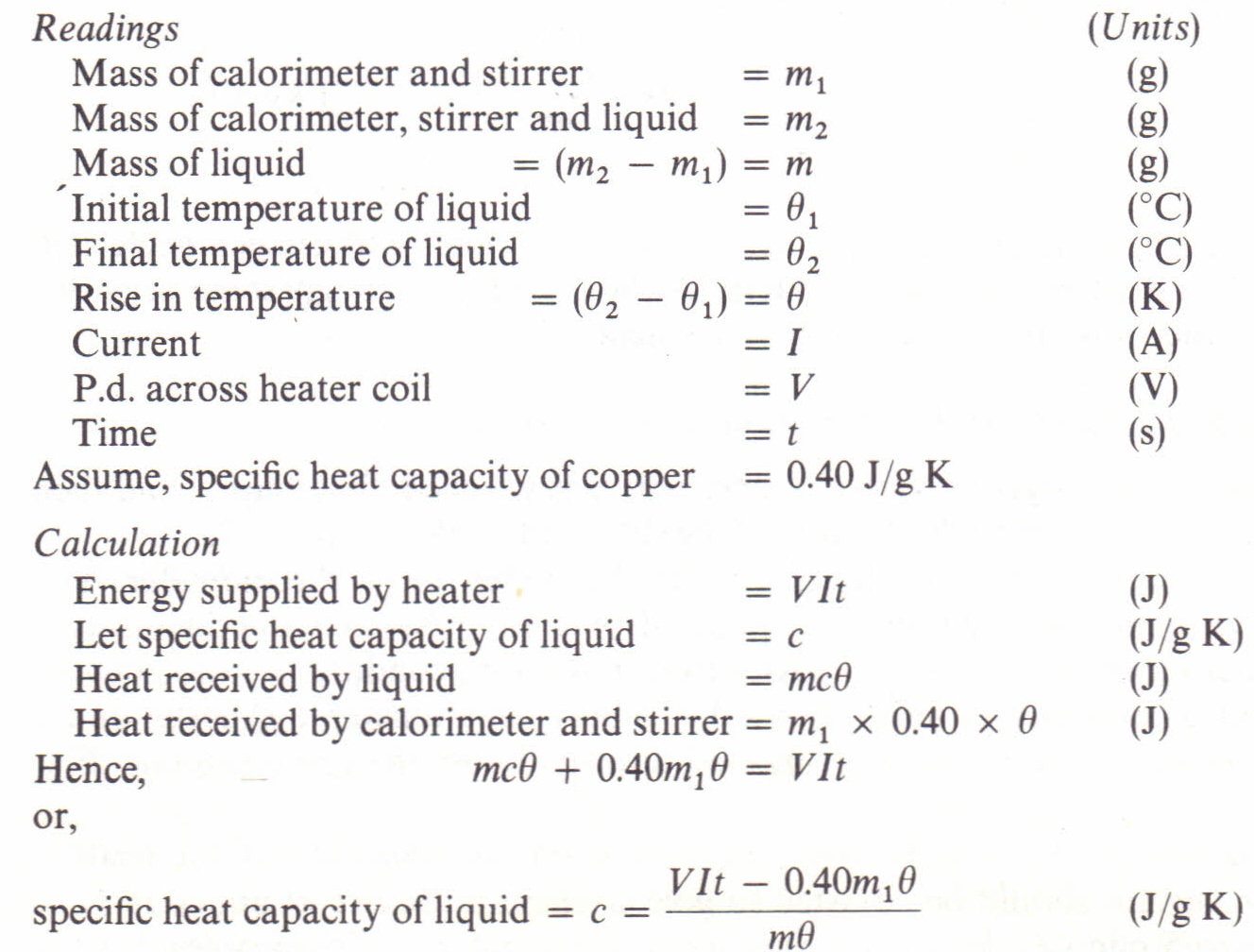
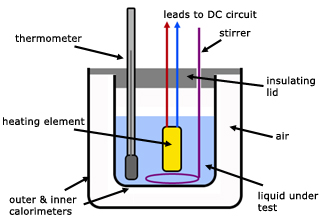
To measure the specific heat capacity of a liquid Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online

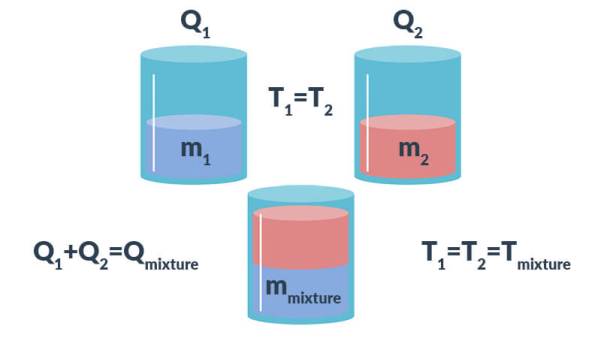
200 g of hot water at 80^∘C is added to 300 g of cold water at 10^∘C . Neglecting the heat taken by the container, calculate the final temperature of the mixture

To measure the specific latent heat of ice by the method of mixtures Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online

To measure the specific heat capacity by the method of mixtures Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online